Satio is pleased to announce a contract awarded by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services to develop a novel, single-use, rapid ebolavirus diagnostic.
This groundbreaking diagnostic will combine Satio’s patch-based blood collection device with Institut Pasteur de Dakar’s sensitive and rapid immunoassay for ebolavirus in a single device. Community health workers can easily deploy such a rapid and sensitive test in remote settings, thereby quickly identifying and treating infected individuals.
By moving away from fingerstick blood samples and associated offboard blood sample manipulation, Satio’s innovation limits exposure to blood borne pathogens while developing a unique low-cost diagnostic that can be easily deployed at the point of need. This is key to improving patient outcomes and preventing the spread of ebolavirus.
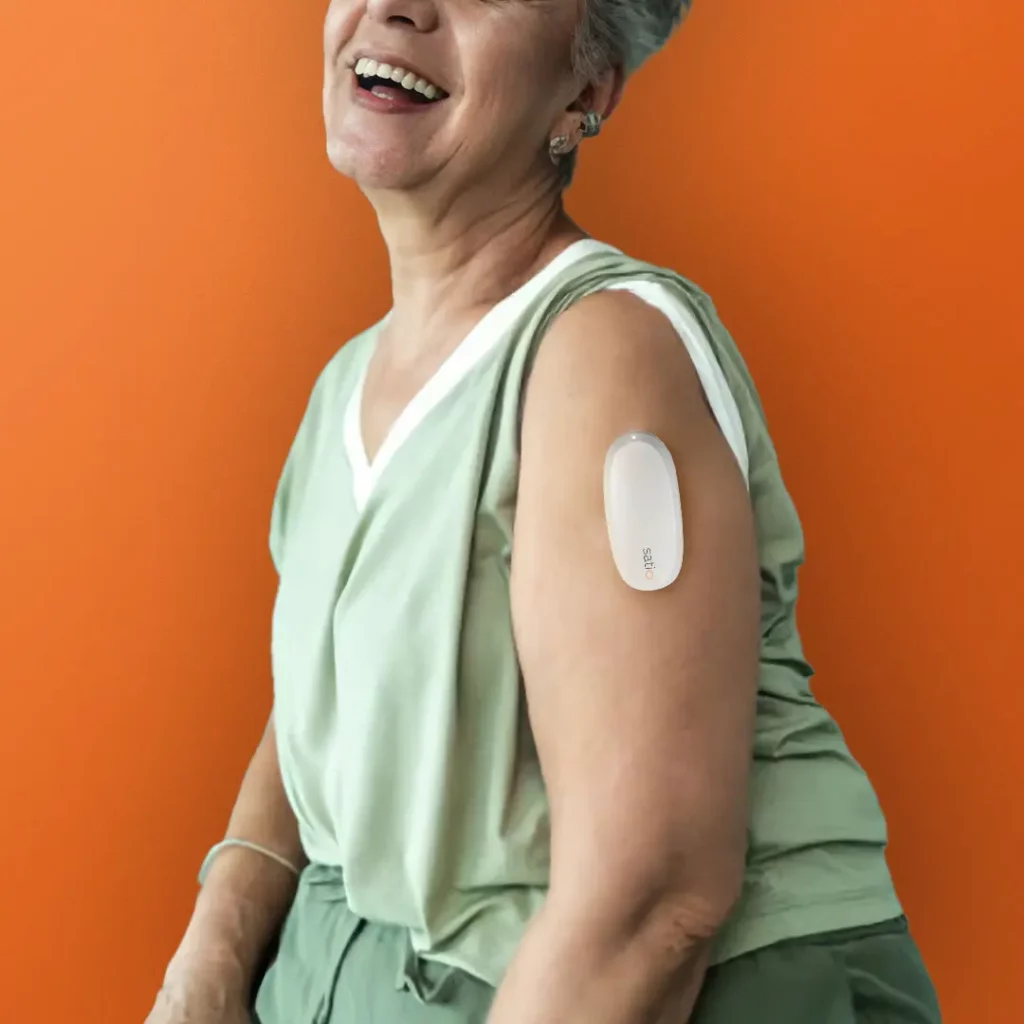
Satio is developing a suite of patch-based platforms including SatioDotTM for dried blood spots, and SatioDrawTM for whole blood collection to enable a wide variety of lab-based testing. These platforms use a lancet to collect blood from the upper arm into the patch. With this funding from BARDA’s Division of Research, Innovation and Ventures (DRIVe), Satio will accelerate the development of an integrated blood draw and diagnostic platform, which can be expanded to detect other important blood-borne pathogens, such as HIV and syphilis.
To ensure that the platform technology is low-cost and accessible to LMICs, Satio will partner with Sapphiros to manufacture the diagnostic on Sapphiros’ innovative and proprietary extreme volume manufacturing process.
Namal Nawana, Executive Chairman and Founder of Satio said, “Satio’s patch-based blood draw, diagnostic, and drug delivery platforms are designed to transform workflow in healthcare. This partnership between BARDA DRIVe, Institut Pasteur de Dakar, Sapphiros, and Satio allows us to develop low-cost, high-performance diagnostics to respond rapidly to biosecurity threats, such as ebolavirus.”
This project has been supported in whole or in part with federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA), under contract number 75A50123C00043.
About Satio
Satio, Inc., is a privately held medical device company focused on point-of-care patches with on board diagnostic and therapeutic solutions leveraging low-cost and user-friendly technology. The company is developing three different platforms. The first is a vaccine and drug delivery patch that allows for intradermal delivery. The second is a dry blood spot and whole blood sampling patches that allows for a wide variety of lab-based and genomic testing. The third is consumer-based diagnostic patch.
About Sapphiros
Sapphiros, LLC, backed by KKR and Neoenta, is a privately held consumer diagnostics company. Sapphiros’s portfolio of capabilities and technologies includes novel sample collection, next generation diagnostics, computational biology, and printed electronics, which help consumers access important diagnostic results globally. Knowing Now Moves Us™
For more information, contact Satio at sjoshi@satiopatch.com