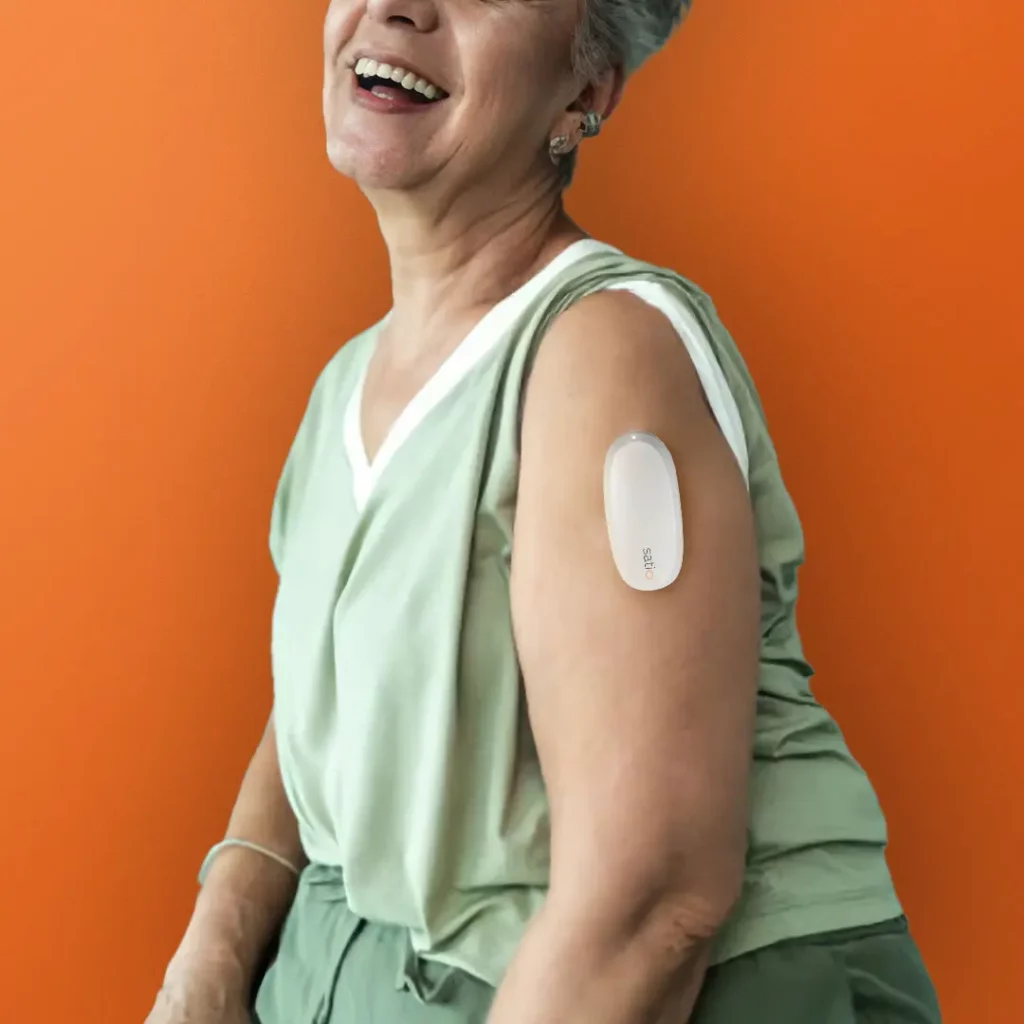
Satio demonstrated novel remotely-controlled telehealth drug delivery automating intradermal delivery and increasing payload 2.5-5x from what is currently typical.
Doing the Hard Stuff.
Rising to an ARPA-H moonshot, Satio created and demonstrated the first remotely-controlled telehealth drug delivery with the additional challenges of automating intradermal delivery (to the thin but richly bioavailable dermis) and increasing payload 2.5-5x from what is currently typical. See some highlights from the day below.
Innovation often begins with a challenge that seems impossible.
“Thank you Ross [ARPA-H], for making us do the hard stuff.”
Breaking Barriers in Remote Care
The project’s ambition sounded like science fiction: “Remote Controlled Tele-injection, straight to EHR… very Star Trek.”
Convenient delivery to the Body’s own biological barrier
Many injections are Intramuscular jabs. “1 ml intradermal injection – at home! It’s just not done.”
Overcoming the Catch-22
“As Ross wrote in his MBA paper, Nobody does it because nobody has it. But nobody has it because nobody does it.” That captures why intradermal is enticing but neglected: the transport didn’t exist, so no one developed the cargo – and vice versa.
Necessity is the Mother of Invention
“To get into that one thin layer, it forced us to do the engineering of a reusable device and then lean into the fact you’ve got motors.” Solving that “hard stuff” also solved for and leapfrogged existing solutions. “By solving this hard stuff, … we can do subcutaneous. And have the patient push the button herself.”

Tapping into the Functionality and Optionality of a Powered Reusable with Stable, Controlled Delivery
One of the key advantages of incorporating a motorized system into a reusable patch device is the ability to achieve precise, stable delivery. As one expert noted, “With a motor, you have this ability to deliver just enough.” This enables that dosing to be carefully managed, avoiding the risks of over-delivery, pain or inconsistent performance.
Equally important is the convenience for patients. The patch format allows for a simple, almost invisible routine: “Because it is a patch… the patient sets it and forgets it… 15 seconds or five minutes.”
Beyond convenience, the integration of a motor with patch technology offers fine-tuned control over delivery pace. As described, “With motor and patch, you can set just the right pace, preset or an algorithm… without pain or drug leakage.”
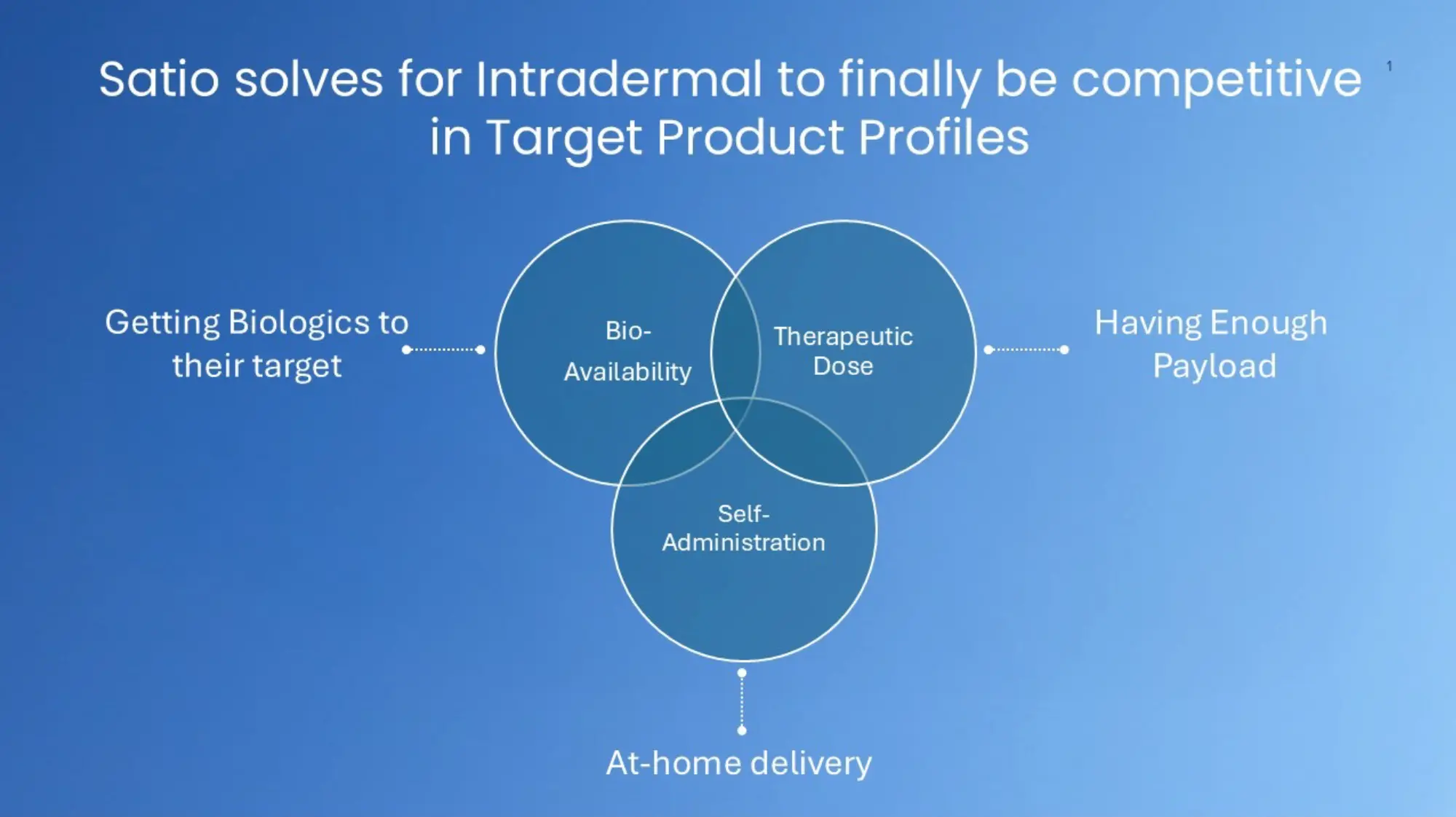
1. Bioavailability: The Recognized Promise of Intradermal Delivery
By accessing the skin’s dense immune, vascular, and lymphatic networks, it can optimize the therapeutic effect of drugs and vaccines. Dose-sparing. Potentially frequency-sparing and even therapy-enabling.
2. Delivering a Therapeutic Payload
At just 0.2–0.4 ml, historical solutions fall short of being worth developing.
3. Self-Administration at Home
TPPs and the market now expect at home. Mantoux is a non-starter, Intramuscular is last resort

Boston’s Unique Role in Driving Health Innovation
“You are sitting right now in the best example of why this is a special place.”
When Brian Johnson delivered this statement, he captured the essence of Boston’s unmatched position in advancing healthcare and technology. Each of these fields—devices, wearables, pharma, software, and regulatory—are vital on their own. Yet, in Boston, they intersect.

Joseph Sullivan, Chief of Strategy & Public Affairs, Massachusetts Life Sciences Center
Namal Nawana, Executive Chairman & Founder, Satio
Ross Urich, DMD, MBA, Program Manager, Health Science Future ARPA-H
Peter Stebbins, Vice President of Program & Business Development, Satio
Brian Johnson, President, MassMEDIC
These products referred to in this article are not FDA approved for sale in the US nor approved in any other territories.
This project has been funded in whole or in part with Federal funds from the National Cancer Institute & Advanced Research Project Agency for Health ARPA-H, National Institutes of Health, Department of Health and Human Services, under Contract No. 75N91023C00059
For more information, contact Peter Stebbins, Vice President Program and Business Development, Satio at pstebbins@satiodx.com.